Abstract
Background: Multiple myeloma (MM) is a clonal plasma cell malignancy that, in most cases, is preceded by an asymptomatic stage termed monoclonal gammopathy of undetermined significance (MGUS) or Smoldering MM (SMM). LAG3 inhibitory receptor is expressed on regulatory plasma cells in mice, and its expression has also been shown to be increased on T cells in SMM and overt MM. Objective: To assess the possible role of LAG3 expression on regulatory plasma cells in MM development. Patients and Methods: Purified plasma cells from bone marrow aspirates (BMA) obtained from patients (pts) with MGUS, SMM, newly diagnosed (NDMM), relapsed-refractory RR (RRMM), and healthy controls were analyzed for the expression of LAG3 by flow cytometry. Autologous cytotoxic peripheral blood T cells were cocultured with LAG3+ or LAG3- plasma cells for 24 hours, while granzyme (Grz) and perforin (Prf) expression in CD8+ T cells was assessed by flow cytometry. Supernatants of cocultures were cryopreserved and further analyzed by Luminex multiplex performance assay for the expression of pro and anti-inflammatory cytokines and vascular endothelial growth factor (VEGF).
Results: Eighty-one patients with a mean age of 70 (44 males and 36 females) from three medical centers in Israel (Bnai Zion, Kaplan, and Galilee Medical Center) were included in the study; 69 were assigned to the experimental cohort, and 12 to the control.
All pts were evaluated by local hematologists based on revised IMWG criteria and assigned to one of four groups: MGUS (17 pts), SMM (20 pts), NDMM (27 pts), or RRMM (5 pts). Twelve patients had normal BMA and served as controls. For further statistical analysis, three groups were defined based on clinical relevance: 1) a control group, 2) a group of patients with premalignant conditions (MGUS and SMM), and 3) a group with overt MM (NDMM and RRMM).
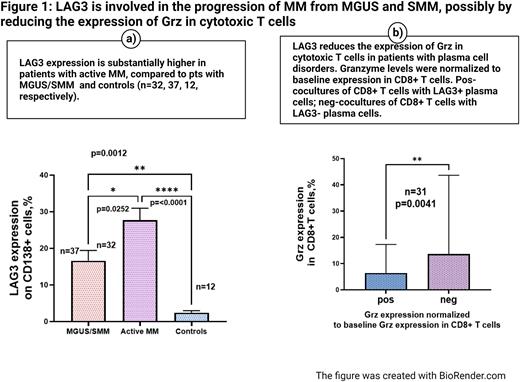
LAG3 expression on plasma cells in active MM (NDMM and RRMM, n=32) was significantly increased compared to its expression on plasma cells in the control group, n=12 (27.69 % ± 3.273 vs. 2.441 % ± 0.517 %, mean ± SEM, respectively, p=0.0001). It also was significantly increased compared to plasma cells collected from MGUS and SMM, n=37(27.69 % ± 3.273 vs. 16.58% ± 2.854, mean ± SEM, respectively, p=0.0252). (Figure 1, a)
The biological role of surface expression of LAG3 on plasma cells was assessed in short-term cocultures of autologous CD8+ T cells either with LAG3+ or LAG3- plasma cells. First, the comparison between LAG3+ and LAG3- cocultures was performed on the entire study population, including patients with premalignant conditions (MGUS and SMM, n=17) and active MM (NDMM and RRMM, n=14). After that, a subgroup analysis was carried out.
Grz expression was significantly decreased in CD8+ T cells cocultured with CD138+/LAG3+ plasma cells compared to cocultures with CD138+/LAG3- cells in the study population, n=31, p=0.0041. (Figure 1, b). In the sub-group analysis, this reduction in Grz was also consistently seen in patients with active MM (15.29% ±5.682 vs. 7.938 % ± 2.409, mean± SEM, respectively, n=14, p=0.0107); it did not reach statistical significance in patients with MGUS/SMM.
The supernatants of the cocultures were also analyzed for the expression of Inf- γ, IL-10, IL17, TNF-α, IL6, IL12, PDL1, and VEGF. Eight patients participated in this experiment (four with active MM and four with MGUS or SMM). Three cytokines (TNF-α, IL6, and VEGF) were detected by multiplex analysis.
VEGF secretion was significantly decreased in supernatants of CD8+ T cells cocultured with LAG3+CD138+ plasma cells compared to cocultures with LAG3-CD138+ cells (9.7± 3.567 vs. 19.67± 4.524, mean ± SEM, p=0.0156). In addition, there was a trend toward a decrease in two other pro-inflammatory cytokines- IL6 and TNF-α.
Conclusion: LAG3 expression on malignant plasma cells may be involved in the progression of MM from premalignant conditions and possibly is linked to a decreased functional capacity of cytotoxic T cells.
Disclosures
Kreiniz:Novartis: Consultancy; Roche: Consultancy. Tadmor:Janssen: Research Funding; AbbVie, Roche, Novartis, Sanofi, Takeda, Janssen, Pfizer: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal